Which of the Following Would Be More Reactive Than Magnesium
Metal at the top is more reactive than the metal at bottom. Potassium atom is larger comparedto that of magnesium and the electronegativityof potassiumis less thanthe magnesium.
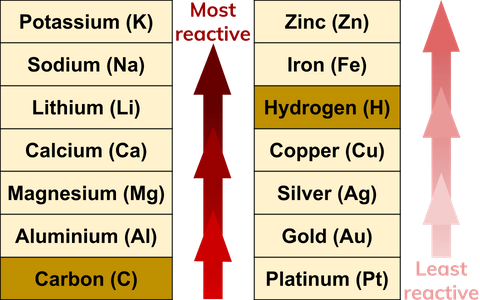
What Are Reactivity Series Definition From Seneca Learning
Calcium is more reactive than magnesium because calcium atom is larger than magnesium atom and it has one more energy level.

. Correct answer - Which of the following would be more reactive than magnesium MG A argon Ar Bberyllium be C CalciumCa D Potassium K. Which of the following would be more reactive than magnesium MG Chemistry. Answerpotassium is more reactive than Mg because both lie in the same group and the element potassium has more electropositivity than magnesium.
So only the following statements are true. The basic range on the pH scale is always greater than 7. Since the concentration of hydroxide ions is greater than that of protons it causes the water to be basic.
2 question Which of the following would be more reactive than magnesium Mg. Lions 14K 8 months ago. A Sodium in more reactive than magnesium.
Calcium is more reactive than magnesium because it is largerthan a magnesium atom because it has one more energy level. Calcium is more reactive than magnesium. 2 on a question Which of the following would be more reactive than magnesium Mg.
C Gold is least reactive. Potassium and Magnesium belongs to the different group. Mg has three outershells because of that its electrons are closer to the nucleus of atom and requires more.
Calcium is more reactive than magnesium because the calcium atom is larger than. These metals are less reactive than the neighboring alkali metal. Determine the identity of a cube of metal that measures 15 cm on each side and has a mass of 3545g.
You might be interested in. Which metal is more reactive calcium or magnesium. This is because when you make a solution of potassium hydroxide it leads to an excess of hydroxide ions in the water.
B Magnesium and aluminium metals displace lead. Why is magnesium less reactive than sodium. The answer is D pH greater than 7.
Valenceelectrons are on the outermost occupied energy level in the atomand. The alkaline-earth metals tend to lose two electrons to form M 2 ions Be 2 Mg 2 Ca 2 and so on. Which of the following would be more reactive than magnesium MG A argon Ar Bberyllium be C CalciumCa D Potassium K.
The reactivity decreases down the line ie. Calcium because its atom is larger. The reactivity of an elementmainly dependsupon the shape and strength.
In displacement reaction more reactive metal displaces the less reactive metal. PotassiumKis the most reactive element than magnesium. Answer 1 of 2.
Which metal is more reactive than magnesium.
What Is The Reactivity Order Of Na K Li And Mg With Water Quora

Gcse Chemistry Reactivity Series Revision 1 Gcse Chemistry Science Revision Chemistry
What Is The Reactivity Order Of Na K Li And Mg With Water Quora
No comments for "Which of the Following Would Be More Reactive Than Magnesium"
Post a Comment